Terpenes are aromatic compounds found in many plants, notably hemp, where they are produced in the trichomes. There are several types. However, for this article, we will focus on just one: limonene. Let’s look at it in detail below. Presentation and Nomenclature Before delving into technical details, it would be helpful to begin with a simple identification of limonene. Therefore, we will focus on its definition and the explanation of its name.So, how can we define it? This article presents it as follows:
Sommaire
ToggleLimonene (C10H16) is a terpene hydrocarbon found in many essential oils, from which it can be obtained by distillation. At room temperature, it is a colorless liquid with a bright, fresh, and clean orange scent, characteristic of citrus fruits. Limonene is notably used in perfumery. Source: https://fr.wikipedia.org/wiki/Limon%C3%A8ne
This same source further specifies that it: (…) is a chiral molecule, and, as with many chiral molecules, biological sources produce a specific enantiomer. The main industrial source, the orange, contains
D
-limonene (+)-limonene, which is the R dextrorotatory enantiomer. Eucalyptus and peppermint, on the other hand, containL(–)-limonene,which is the S
levorotatory enantiomer. Racemic limonene is known as
dipentene.
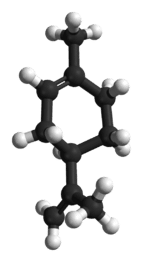
Source: https://fr.wikipedia.org/wiki/Limon%C3%A8neIn addition to the presentation of the terpene, as given above, it would be useful to briefly focus on its nomenclature. In fact, what is the origin of the term limonene ? For those in the know, this word implicitly evokes the word “lemon.” A coincidence? Certainly not! Why? Our source indicates that this name comes from: (…) the name of the lemon, which, like other citrus fruits, contains considerable amounts of this chemical compound, largely responsible for its fragrance. Source: https://fr.wikipedia.org/wiki/Limon%C3%A8neChemical Considerations After its brief introduction, let’s examine how to define this terpene chemically. In this regard, we propose to focus on the following points: structure, identification, chemical properties, physical properties, and biosynthesis. The Structure of R-LimoneneIn chemistry, its structure is as follows.
limonene
Credit: Wikipedia Identification The following elements allow for its identification.
IUPAC Name:
1-methyl-4-prop-1-en-2-yl-cyclohexene
No.
CAS: 5989-27-5 ( R) 5989-54-8 (
S
)
No.
- ECHA: 100.028.848 No.
- EC: 227-813-5 (R ) 227-815-6 (–)PubChem: 440917FEMA: 2633Appearance: colorless liquid with a characteristic odor (d-limonene).Chemical PropertiesBelow are its chemical properties.
- Chemical formula: C10H16 Molar mass: 136.234 ± 0.0091 g/mol
- C 88.16%, H 11.84%Physical properties Below are its physical properties.Melting point: −75 °C (d-limonene)
- Boiling point: 176 °C (d-limonene)
- Solubility: in water: none (d-limonene)
- Density: 0.84 g·cm⁻³ (d-limonene)
Auto-ignition temperature: 255 °C
Flash point: 48 °C (d-limonene)
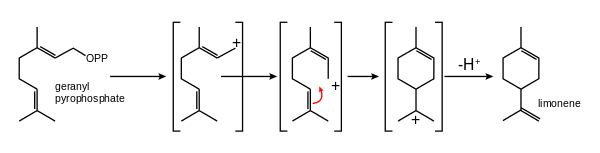
- Explosive limits in air: 0.7–6.1% volSaturated vapor pressure: at 14.4 °C: 0.4 kPa (d-limonene)BiosynthesisIndeed, it is formed from geranyl pyrophosphate, through the cyclization of a neryl carbocation or its equivalent. Here is a representation of this biosynthesis.
- Limonene
Credit: Wikipedia
Health considerations
- As with any molecule or chemical component, it’s always important to consider its health implications. That being said, we note that, overall, this terpene is not dangerous. How can this be explained? In fact, it’s used in the food and pharmaceutical industries to flavor food and medications. Similarly, some cleaning products use it for its refreshing scent.
- However, long-term use in cleaning products can have side effects in some people, notably skin irritation.
- Regarding toxicity: Limonene has been fully approved as a safe food additive and flavoring by the Food and Drug Administration since 1994! During the toxicity assessment of the compound, they found that it had “relatively low acute toxicity via oral administration.”
- This terpene, limonene, is naturally present in CBD flowers.
- Also read about CBD. Linalool
- Nerolidol
Terpinolene
Terpenes